Henry Cavendish: the Man Who Discovered Hydrogen
- Georgina Griffiths

- Jan 12, 2021
- 3 min read

Personal Life:
Henry Cavendish was born 10th October 1731 to a wealthy and aristocratic family. He went to Newcome's school near London and then later attended Cambridge at Peterhouse college (formerly called St. Peter's) which he left after only three years without a degree. He would then move in with his father and would mainly reside in his laboratory there in the forthcoming years. Henry's father, Lord Charles Cavendish, was initially in politics but would soon spend more and more time on science and would become a member of the Royal Society in London. Lord Cavendish would take Henry to dinners and other events at the society and in 1760 he would become a member of the society and would even be elected a member of the council in 1765.
Henry Cavendish was well known to avoid most social events and would spend most of his time alone with his science. He never married and would generally only socialise with other scientists. He was known to wear an old-fashioned suit and only had close relationships with those in his own family.
Following his fathers death Cavendish spent a great deal of time with a fellow scientist called Charles Blagden and their mutualistic relationship helped each other as Cavendish helped Blagden enter into London's scientific society. Which Blagden then assisted Cavendish in return by preventing him from having to be involved in the society which he disliked so much.
The Discovery of Hydrogen:

Although Hydrogen had successfully been produced and isolated(as pictured to the left) by other scientists through reacting reacting metals with acid, Cavendish managed to accurately note hydrogen as a unique element. Due to the contemporary lack of understanding about gases he described two types of air, inflammable (hydrogen) and fixed air (carbon dioxide), the latter being produced by reacting acids with alkalis. Upon this discovery he then accurately calculated that in water hydrogen atoms were proportioned two to one. This was done through burning a known quantity of hydrogen in air and then measuring the quantity of the newly formed water from this reaction. (This is the point where I should note that Cavendish also successfully managed to recognise that burning hydrogen causes water to form) He also concluded that the gas released through respiration was fixed air, or carbon dioxide as we would call it today. Cavendish would be awarded the Copley medal by the Royal Society for his studies on the gases in the air.
Cavendish would continue to study the makeup of the gasses in the air and would conclude that the oxygen to nitrogen ratio in air was one part oxygen to four parts nitrogen. This is actually very accurate and the current known ratio is one part oxygen to 3.7 parts nitrogen. He calculated this by combining a known volume of Hydrogen and air and then the mixture was exploded with a spark of electricity. He also calculated that once he knew the volume of nitrogen and hydrogen there was a gas remaining that was 1/120 of the original volume of the nitrogen, Cavendish believed this to be a calculation error but roughly 100 years later two physicists, William Ramsay and Lord Rayleigh, would be able to point out that this was the inert gas which they had recently discovered, argon.
Calculating the Density of the Earth:
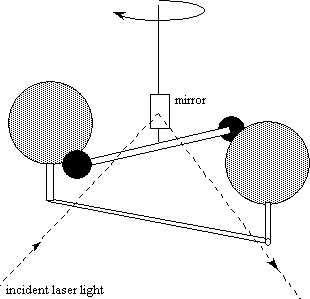
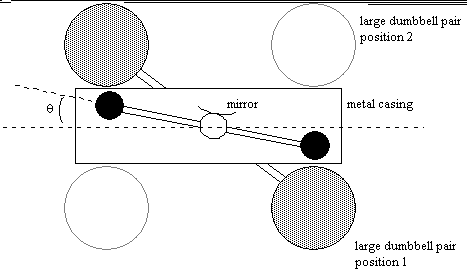
Alongside his discoveries in the gasses of the atmosphere Cavendish also worked on calculating the density of the earth and was also fairly successful. He did this by suspending two small lead balls from the arms of a torsion balance and then placing two larger lead balls. By measuring the angle that the arm with the smaller lead balls is to the axis you can calculate the attraction between the smaller balls with the larger ones and from the attraction between the balls you can calculate "G" or the gravitational constant. The Harvard University website explains this experiment far better than I can so I do suggest checking that first (you can find the link in the references section at the end of the page). This revolutionary experiment would become known as the Cavendish experiment.
References:
"Henry Cavendish", from Britannica, accessed 23/12/2020 https://www.britannica.com/biography/Henry-Cavendish
images via "Henry Cavendish", from Wikipedia, accessed 23/12/2020 https://en.wikipedia.org/wiki/Henry_Cavendish
"Henry Cavendish and The Revolutionary Discovery of Hydrogen", from Interesting Engineering, accessed 22/12/2020 https://interestingengineering.com/henry-cavendish-and-the-revolutionary-discovery-of-hydrogen
"When Did We Discover that Hydrogen Produces Water when Burned? | Earth Lab", by Brian Cox, video from the BBC, https://www.youtube.com/watch?v=zQaYLbsl33g
"The Cavendish Experiment", from Harvard University https://sciencedemonstrations.fas.harvard.edu/presentations/cavendish-experiment



Comments